and Indianapolis have been at the center of insulin production and innovations in diabetes care since immediately after the hormone was discovered in 1922 at the University of Toronto (Canada). Insulin is secreted in the pancreas and is essential to the metabolism of carbohydrates and fats. Its deficiency leads to the development of diabetes. Many diabetics require regular injections of insulin to maintain life and health.
When the Canadians had difficulty making more than small laboratory batches of the hormone, they accepted an offer of collaboration from , the research director of Eli Lilly and Company. Under a formal agreement signed in May 1922, Lilly and the University of Toronto fully shared their knowledge of insulin, with the company being given a one-year exclusive license for the U.S. market.
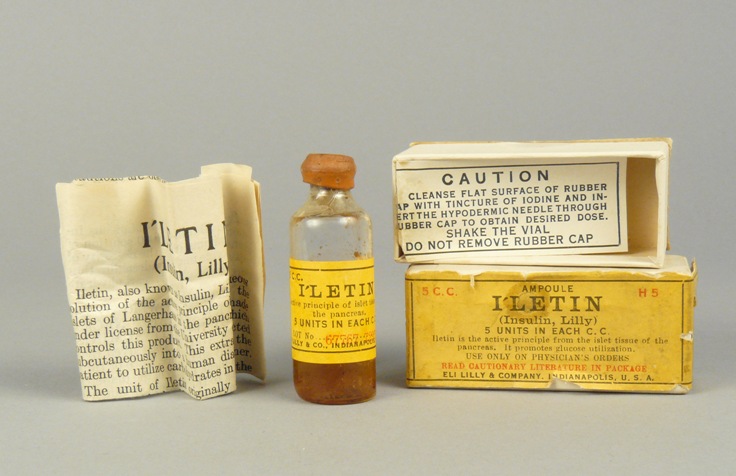
The focus of insulin production immediately shifted to Indianapolis. The Lilly product, Iletin, initially prepared from beef or hog pancreas, was exported to Canada and used in clinical trials that signaled a milestone in treatment for millions of diabetics. Demand exceeded the supply until the spring of 1923 when a large-scale apparatus was placed in operation. The volume of production then increased dramatically. On October 15, 1923, Lilly insulin was released for distribution by physician prescription. By the close of the year, Lilly had sold almost 60 million units. A total of 10 price reductions occurred between 1923 and 1936.
Eli Lilly and Company subsequently made important advances in insulin isolation and purification and used its head start to dominate the U.S. market for insulin, a position it has never relinquished. Insulin has been the most important product in the company’s history and Lilly has continued to be a world leader in insulin research.
In 1982, Lilly introduced synthetic human insulin (Humulin), made by techniques of genetic engineering, marking another milestone in the history of science and therapeutics. The company had partnered with Genentech to produce human insulin made from recombinant DNA technology. Together, Lilly and Genentech scientists were able to produce large quantities of biosynthetic human insulin. Health care providers quickly realized the benefits of human insulin, which was less likely to form antibodies or cause localized reactions in patients. Human insulin became the gold standard of insulin treatment for diabetes. In the 1990s, the company introduced Humalog, a fast-acting insulin product.
In the 21st century, Lilly has continued to put Indianapolis on the map as a leader in the production of drugs to treat diabetes. In January 2011, Lilly and Boehringer Ingelheim, a German pharmaceutical company, made an agreement to jointly develop and market new diabetes therapies. The alliance resulted in the development of Tradjenta, a prescription drug used to lower blood sugar in adults with type 2 diabetes in combination with diet and exercise.
In 2014, Lilly launched Jardiance and Trulicity, both designed for the treatment of type 2 diabetes. In 2019, the FDA approved Lilly’s Nasal Glucagon to treat severe hypoglycemia. It provides a simple one-step delivery system that saves crucial time from the multistep system that it replaced, which prevents hospitalization of diabetes patients who are four years old or older in emergency situations. The company has several other diabetes-care products in its research and development pipeline.

Help improve this entry
Contribute information, offer corrections, suggest images.
You can also recommend new entries related to this topic.